Archive
03. October 2022
Effectum Medical a supporter of the Swiss healthcare ecosystem

Swiss Healthcare Startups is a non-profit organisation that supports innovative startups that aim to add value to the Swiss healthcare ecosystem. The organisation supports startups by providing meaningful exposure and facilitating access to relevant stakeholders thanks to an exclusive digital directory, an extensive partner network, and by organising some of the most inspiring events in Swiss healthcare.
Healthcare innovation is at the heart of Effectums’s business and as an institutional member of Swiss Healthcare Startups we look forward to supporting the Swiss Healthcare ecosystem.
29. August 2022
ISO 13485 Certificate for In-Vitro Diagnostic products received

In spring, we completed our first IVD audit and our ISO 13485 certificate for In-Vitro Diagnostic products has now arrived! This has been the next important step for us to complete our service offering which now covers In-Vitro diagnostics in addition to Medical Device products and software. We are of course pleased to have received the certificate, but we are equally happy to have team members with a proven track record in diagnostics who can expertly accompany our customer projects.
28. July 2022
Swiss Symposium in Point-of-Care Diagnostics October 20th, 2022

This years POCDx Symposium will take place in Basel and Effectum Medical is honored to be a part of it. In addition to the main symposium there will be a pre-event held on October 19th where Effectum Medical will be speaking about IVDR Requirements for Diagnostic Products – in vitro tests, software, instruments
Registration is now open and you can benefit from early bird rates until August 31st, 2022.
For more details about the symposium and to register please visit the symposium website.
14. March 2022
Welcome to the team

We are pleased to announce that Harald Züger has joined the Effectum team. He is taking care of our financials and bringing them to the next level. Harald is a very skilled finance executive with 20+ years of comprehensive international, management and board experience. Since 2010 he has been providing finance and operational support for life science and healthcare start-ups in Switzerland, France and Austria and has worked in set-ups like Effectum before.
For more information about Harald visit his LinkedIn profile.
31. January 2022
5 year anniversary Effectum Medical
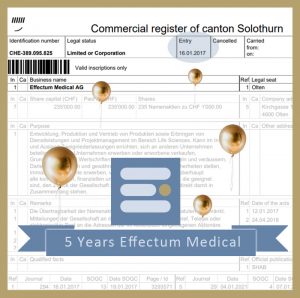
5 years ago, on January 16th, 2017 Effectum Medical was officially born. A big thank you to our great customer, partners and team without whom this journey would not be possible!
(more…)
09. December 2021
Effectum Medical joins the KAPSLY service-for-equity platform

Supporting start-ups is in our DNA. Therefore, we are very happy to announce that we have joined the KAPSLY platform! KAPSLY is a service-for-equity platform that connects service providers with promising start-ups.
We are the right partner to validate product concepts through the lens of regulatory requirements. Through our sister company MEDICALBOARD we can also offer Marketing and Strategy expertise and are thereby able to support start-ups throughout their product journey.
For more information about KAPSLY visit their website or get in touch!
23. April 2021
We are hiring
Effectum Medical is a young company that is breaking new ground in the areas of quality management and regulatory affairs in the medical device industry. Our vision is to facilitate and accelerate medical device product innovation by offering an affordable, outsourced quality management system (QMS) and legal manufacturing. Our customers are start-ups, as well as global medical device manufacturers.
To further grow our company, we are looking for a
Junior Project Manager Medical Devices – 80-100%
to join our team.
(more…)






