
Are you a manufacturer domiciled outside of Switzerland and want to sell products on the Swiss market?
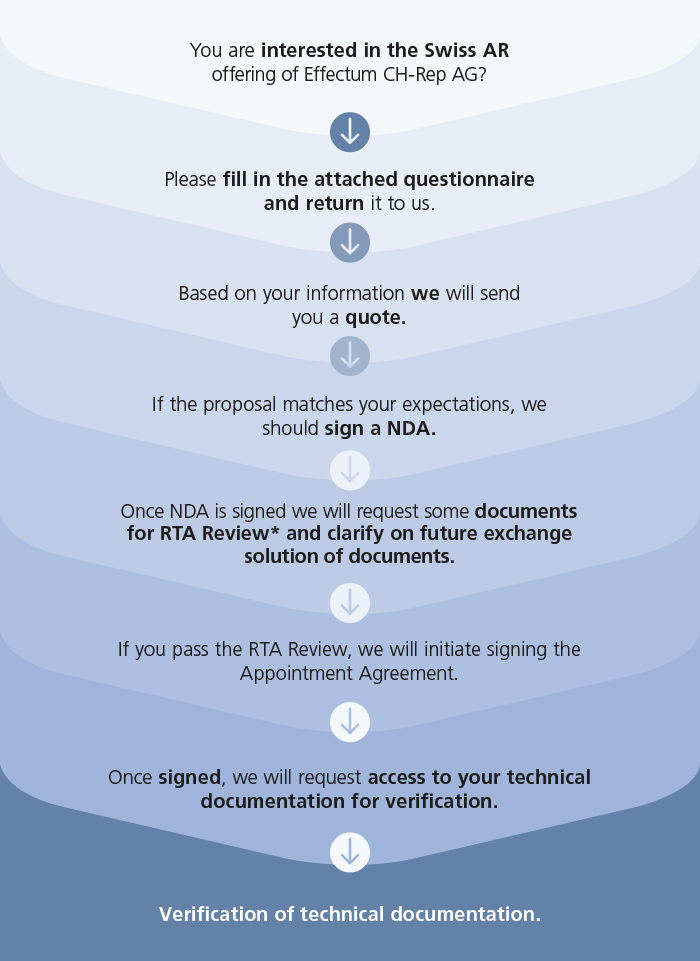
Share a few information with us for a quote.
immediate information about complaints and reports from healthcare professionals, patients and users
ensure that it has permanently and continuously at its disposal

Our standard services include outsourced Quality Management System and Legal Manufacturer – sharing risk has always been part of our business model
Sound and up-to-date knowledge about MDR/IVDR
In-house PRRC
Liability insurance
Experienced editors and reviewers of technical documentation
ISO 13485
Process lead from ideation until final submission
Senior Team of Regulatory Affairs Managers
Qualified to take over responsibility during product development and market phase, i.e. post market surveillance, vigilance, complaint handling, supplier management, etc.
Existing relations to Notified Body and national authorities